.jpg)

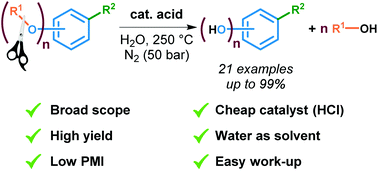
19. Efficient demethylation of aromatic methyl ethers with HCl in water
Jeroen Bomon, Mathias Bal, Tapas Kumar Achar, Sergey Sergeyev, Xian Wu, Ben Wambacq, Filip Lemière, Bert F. Sels and Bert U. W. Maes*
Green Chem., 2021, 23, 1995-2009
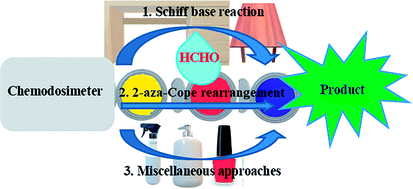
18. Recent advances in selective formaldehyde detection in biological and environmental samples by fluorometric and colorimetric chemodosimeters
Saikat Kumar Manna,* Tapas Kumar Achar and Sanchita Mondal
Anal. Methods, 2021, 13, 1084-1105

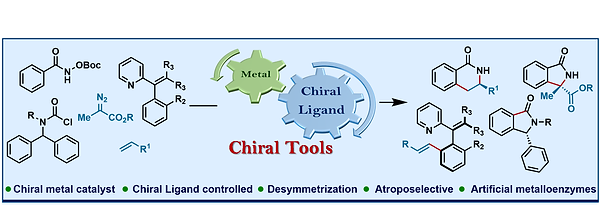

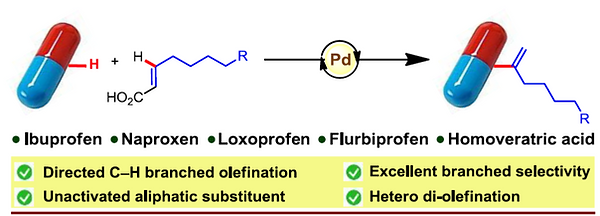










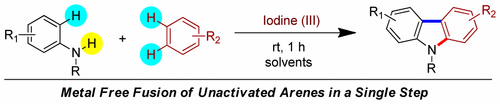

17. Orthogonal Selectivity in C–H Olefination: Synthesis of Branched Vinylarene with Unactivated Aliphatic Substitution
Tapas Kumar Achar, Sudip Maiti, Sadhan Jana and Debabrata Maiti*
ACS Catal. 2020, 10, 13748–13793
Synopsis: Transition metals in combination with appropriate chiral ligands play an important role in controlling the stereoselectivity during C−H activation, thus providing an imperative tool to the synthetic community to grip the control over introduction of a stereocenters in a reaction sequence. This review provides a comprehensive overview of transition-metal catalyzed enantioselective C(sp2)–H Bond functionalization.
16. Orthogonal Selectivity in C–H Olefination: Synthesis of Branched Vinylarene with Unactivated Aliphatic Substitution
Soumitra Agasti, Bhaskar Mondal, Tapas Kumar Achar, Soumya Kumar Sinha, Anjana Sarala Suseelan, Kalman J. Szabo*, Franziska Schoenebeck*, and Debabrata Maiti*
ACS Catal. 2019, 9, 9606–9613
Synopsis: Orthogonal mode of insertion of alkenyl carboxylic acids could lead to branched-selective olefinations overriding Fujiwara-Moritani and Mizoroki-Heck reaction for linear-selective olefination.
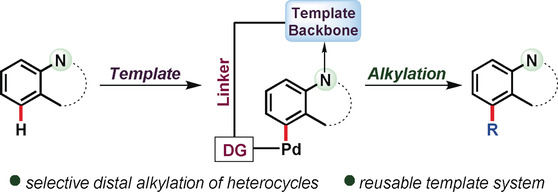
15. Coordination Assisted Distal C−H Alkylation of Fused Heterocycles
Kankanala Ramakrishna, Jyoti Prasad Biswas, Sadhan Jana, Tapas Kumar Achar, Sandip Porey and Debabrata Maiti*
Angew. Chem. Int. Ed. 2019, 58, 13808–13812
Synopsis: Distal C−H alkylation (C‐5 of quinoline and thiazole, C‐7 of benzothiazole and benzoxazole) of heterocycles is reported. Upon complexation with heterocyclic substrate, nitrile DG in template directs the metal catalyst towards close vicinity of the specific distal C−H bond of the heterocycles. The hypothesized pathway is supported by various X‐ray crystallographically characterized intermediates.
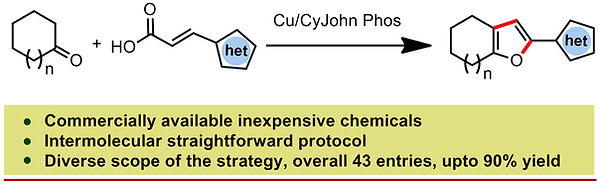
14. Regioselective Synthesis of Fused Furans by Decarboxylative Annulation of α,β‐Alkenyl Carboxylic Acid with Cyclic Ketone: Synthesis of Di‐Heteroaryl Derivatives
Soumitra Agasti, Tapas Pal, Tapas Kumar Achar, Siddhartha Maiti, Debasis Pal, Smita Mandal, Kishan Daud, Goutam Kumar Lahiri* and Debabrata Maiti*
Angew. Chem. Int. Ed. 2019, 58, 11039-11043
Synopsis: A facile CuII‐mediated [3+2] annulation of aliphatic cyclic ketones and alkenyl carboxylic acids leads to synthetically useful fused furan derivatives. The reaction proceeds by a single‐electron transfer and a decarboxylation from commercially available starting materials.
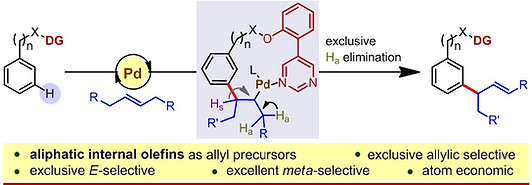
13. Palladium‐Catalyzed Directed meta‐Selective C−H Allylation of Arenes: Unactivated Internal Olefins as Allyl Surrogates
Tapas Kumar Achar, Xinglong Zhang, Rahul Mondal, M. S. Shanavas, Siddhartha Maiti, Sabyasachi Maity, Nityananda Pal, Robert S. Paton* and Debabrata Maiti*
Angew. Chem. Int. Ed. 2019, 58, 10353-10360
Synopsis: Palladium(II)‐catalyzed meta‐selective C−H allylation of arenes has been developed utilizing synthetically inert and unbiased acyclic aliphatic olefins as allylic surrogates. The reactions are exclusively allylic selective and E‐selective, as well as atom economic.
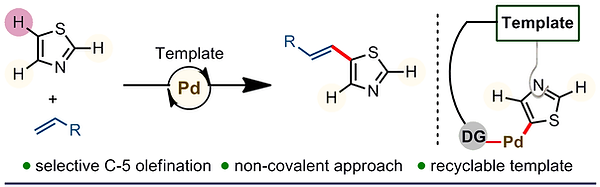
12. Palladium-Catalyzed Template Directed C-5 Selective Olefination of Thiazoles
Tapas Kumar Achar, Jyoti Prasad Biswas, Sandip Porey, Tapas Pal, Kankanala Ramakrishna, Siddhartha Maiti and Debabrata Maiti*
J. Org. Chem. 2019, 84, 8315–8321
Synopsis: A straightforward protocol has been developed to afford C-5 olefinated thiazole derivatives in a highly selective manner utilizing coordinative interaction between the substrates and the metal chelated bifunctional template backbone.
11. Accessing Remote meta‐ and para‐C(sp2)−H Bonds with Covalently Attached Directing Groups
Aniruddha Dey, Soumya Kumar Sinha, Tapas Kumar Achar and Debabrata Maiti*
Angew. Chem. Int. Ed. 2019, 58, 10820-10843
Synopsis: This review gives an overview on utilization of covalently linked directing groups for meta/para-C-H activation of aromatic molecules reported in the last few years.
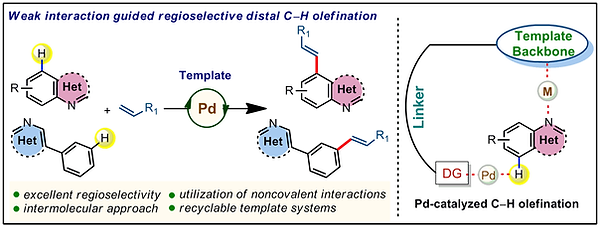
10. Regiocontrolled Remote C−H Olefination of Small Heterocycles
Tapas Kumar Achar, Kankanala Ramakrishna, Tapas Pal, Sandip Porey, Pravas Dolui, Jyoti Prasad Biswas, and Debabrata Maiti*
Chem. Eur. J. 2018, 24, 17906-17910
Synopsis: Non-covalent interactions lead to site-selective remote C−H bond functionalizations of small heterocycles with the assistance of an intermolecularly interacting bifunctional template.
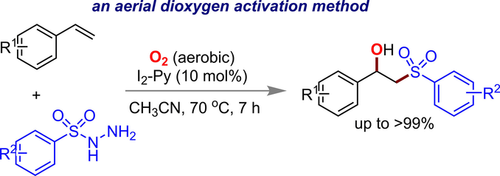
9. Iodine‐Triggered Aerobic Oxysulfonylation of Styrenes
Khokan Choudhuri, Tapas Kumar Achar and Prasenjit Mal*
Adv. Synth. Catal. 2017, 359, 3566-3576
Synopsis: Iodine-triggered aerial dioxygen activation was identified and applied for oxysulfonylation reactions towards the regioselective synthesis of b-hydroxysulfones from vinyl arenes and sulfonyl hydrazides.
8. Mechanochemical synthesis of small organic molecules
Tapas Kumar Achar, Anima Bose and Prasenjit Mal*
Beilstein J. Org. Chem. 2017, 13, 1907–1931
Synopsis: This review highlights the application of mechanochemical synthesis of heterocyclic rings, multicomponent reactions and organometallic molecules including their catalytic applications.
7. An Organic Intermolecular Dehydrogenative Annulation Reaction
Saikat Maiti, Tapas Kumar Achar and Prasenjit Mal*
Org. Lett. 2017, 19, 2006–2009
Synopsis: An I(III)-mediated simple annulation approach has been developed for carbazole synthesis by utilizing two non-prefunctionalized monocyclic arenes via simultaneous functionalization of three C(sp2)−H bonds and one N(sp3)−H bond.
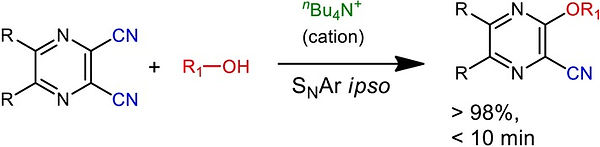
6. Cation‐π Assisted Synthesis of Alkyl Aryl Ethers via C‐CN Functionalization of 1,2‐Dicyano Pyrazines
Tapas Kumar Achar, Prasit Kumar Sahoo and Prasenjit Mal*
Chem. Select. 2017, 2, 1944-1949
Synopsis: The weak cation-π interaction was found important to activate the C−CN bond for aromatic nucleophilic (SNAr) ipso substitution.
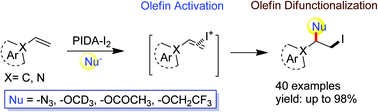
5. PIDA-I2 Mediated Direct Vicinal Difunctionalization of Olefins: Iodoazidation, Iodoetherification and Iodoacyloxylation
Tapas Kumar Achar, Saikat Maiti and Prasenjit Mal*
Org. Biomol. Chem. 2016, 14, 4654−4663.
Synopsis: I+ or IOAc, in situ generated from PIDA-I2 mixture, facilitated the direct vicinal difunctionalization of olefins via cation–π interaction at room temperature and under transition-metal free conditions.
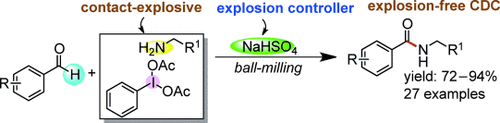
4. Transformation of Contact-Explosives Primary Amines and Iodine(III) into a Successful Chemical Reaction under Solvent-Free Ball Milling Conditions
Tapas Kumar Achar and Prasenjit Mal*
Adv. Synth. Catal. 2015, 357, 3977−3985.
Synopsis: This work highlights mechanochemical cross dehydrogenative coupling (CDC) reactions for the amide synthesis by controlling the explosive nature of primary amine and iodine(III) in constrained media utilizing acid-salt NaHSO4
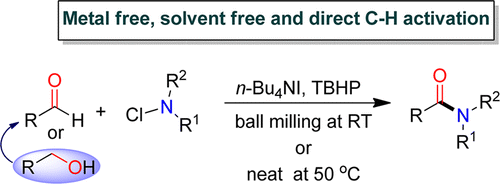
3. Radical-Induced Metal and Solvent-Free Cross-Coupling Using TBAI-TBHP: Oxidative Amidation of Aldehydes and Alcohols with N-Chloramines via C−H Activation
Tapas Kumar Achar and Prasenjit Mal*
J. Org. Chem. 2015, 80, 666−672.
Synopsis: The TBAI-TBHP combination was found to be beneficial for oxidative amidation of aldehydes and alcohols under solvent-free conditions with a series of N-chloramines.
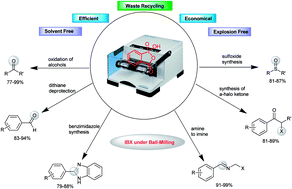
2. IBX Works Efficiently under Solvent Free Conditions in Ball Milling
Tapas Kumar Achar, Saikat Maiti and Prasenjit Mal*
RSC Adv. 2014, 4, 12834−12839.
Synopsis: This work describes an overview of a highly economical synthetic methodology which overcomes the long standing problems of using IBX in various synthetic transformations, efficiently in gram scale and in a safe way avoiding possible explosion risk.
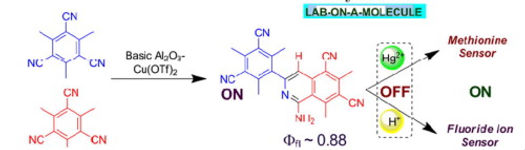
1. An Isoquinoline as Cation Assisted ON–OFF–ON Fluorescence Switch with Methionine and Fluoride ion
Tapas KumarAchar, VedPrakash, Himansu S. Biswal and Prasenjit Mal*
Tetrahedron Lett. 2013, 54, 1067−1070.
Synopsis: A novel method has been developed for the synthesis of 1-amino-3-aryl isoquinoline (IQ) and further utilization of the IQ in ion-sensing properties.


.jpg)